Data Integrity Sop in Pharmaceutical Industry
MHRA -Data Integrity Definitions and Guidance Data Integrity is the extent to which all data are complete consistent and accurate throughout the data lifecycle. All raw data all printed electronic data Chromatogram spectrum weight prints This can be done on a sheetrecord where all results have been summarized If a notebook is no longer used and unused pages are.

Sop On Data Integrity In Pharmaceutical Industry
Real-time Plant Data Sharing Service WebTechnician Profit-driven Operation Digital Plant Operation Intelligence DPI Quality Stabilization System OpreX Data Model Broker Design Data Validation Plant Information Management System OpreX Batch Solution Secured Remote Solutions Security Program Cybersecurity Consulting Services.
. Consistency is particularly important in a regulated environment so as to ensure outcomes that can be relied upon in the long term. Procedure Analysis for SOP Optimization Alarm Behavior Analysis ABA Operator Training System Omega Simulation Product Training Operator Training Simulator Cybersecurity Awareness Training Super Resolution Wide Field of View High Speed Uniformizer CV8000 High-Throughput System Benchtop CQ1 Confocal System Software Advanced Control Bioreactor. As global public health and safety standards rise companies must ensure that their employees receive the necessary education and training to remain competitive.
Authorized designee of IT shall be responsible for preparation of SOP backup restoration of data. 48 Records should be made or completed at the time each action is taken and in such a way that all significant activities concerning the. Good Documentation Practice Review of the original records when the records are complete after complete analysis of a sample has been performed.
It will gives all data required for assessing the batch manufacturing on production scale. The 1120 citations that went out from October 2020 to September 2021 covered more than 200 different categories of noncompliance. Its comes when first three batches of product being manufactured on production scale very closely.
Life Sciences companies are expected to. A Standard Operating Procedure or SOP is a set of step-by-step instructions compiled by an organization to help workers carry out routine operations in a clear and consistent manner. This SOP is applicable for all the computers associated with laboratory other system equipments used manufacturing facility of xxx.
This data collected during batch manufacturing will give precise in depth idea about its fundamentals. Discuss with subject matter expert Review Raw Data analytical method validation report method transfer report and forced degradation data to find the root cause. Based on the above review he has to prepare a hypothesissimulation study protocol and plan the study as per approved protocol to find out the root cause or assignable cause for Out of Specification OOS.
Head IT shall be responsible to review the SOP to ensure procedure is carried out as per SOP. 47 Handwritten entries should be made in a clear legible indelible way. Promotion of a quality culture together with implementation of organisational and technical measures which ensure.
The US FDA releases an annual data summary of inspection observations by industry. Out of those categories more than one-third 32 of the observations fit into 10 categories which tells a clear story about common. Data integrity enables good decision-making by pharmaceutical manufacturers and regulatory authoritiesIt is a fundamental requirement of the pharmaceutical quality system described in EU GMP chapter 1 applying equally to manual paper and electronic systems.
And with the help of trend analysis test results manufacturing process time.
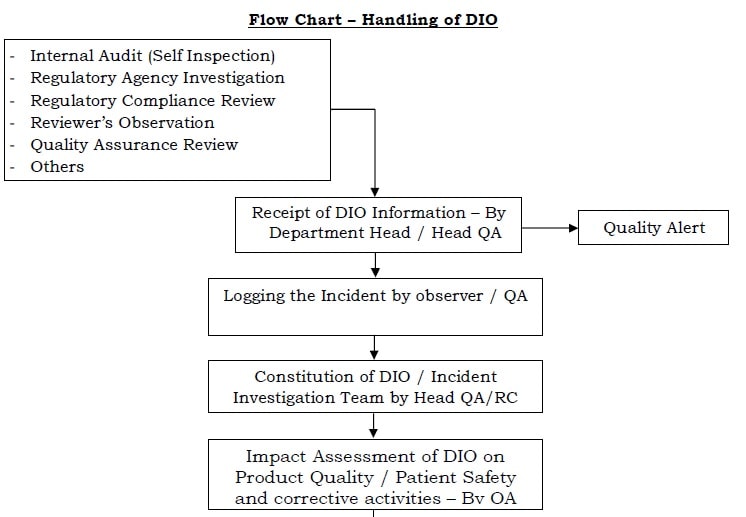
Data Integrity Handling Of Di Observations Dio Pharma Beginners

Data Integrity Handling Of Di Observations Dio Pharma Beginners
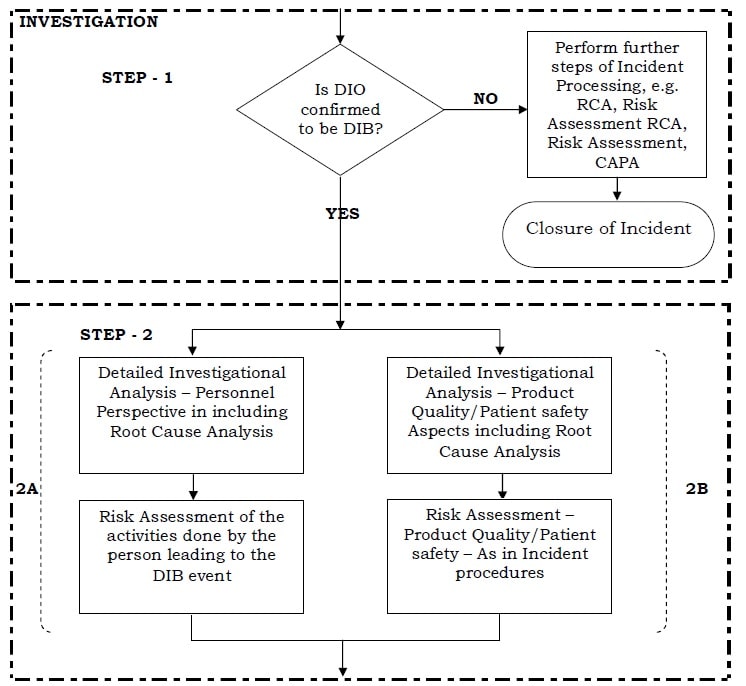
Data Integrity Handling Of Di Observations Dio Pharma Beginners

Comments
Post a Comment